What is Lead Concentrate?
Lead is a dense, soft, low-melting metal. It’s an important component of batteries, and about 75th of the world’s lead production is consumed by the battery industry. Lead is the densest common metal except for gold, and this quality makes it effective in sound barriers and as a shield against X-rays. Lead resists corrosion by water, so it has long been used in the plumbing industry. It’s also added to paints, and it makes a long-lasting roofing material. Lead paint is found in several older buildings, but it’s now mostly used on outdoor steel structures like bridges, to improve their weather ability. A lead compound known as tetraethyl lead was added to gasoline as early as 1921 because it prevented the knocking problem of high-compression automobile engines. However, most gasoline currently contains no lead, because lead from car exhaust was a major source of air pollution.
Lead is also commonly used in glass and enamel. In TV picture tubes and computer video display terminals, lead helps block radiation, and the inner, though not the outer, portion of the common light bulb is created of leaded glass. Lead also will increase the strength and brilliance of crystal glassware. Lead is used to create bearings and solder, and it is very important in rubber production and oil refining. Builders used lead as a roofing material for cathedrals, and lead was additionally wanting to hold together the different panels of colored glass in stained glass windows. Modern lead mines produce about 2 million metric tons of lead annually. This is only about half of the lead used worldwide; the remainder is obtained by use. The top producer of lead is Australia, followed by the USA, China, Iran, and Canada. Other countries with major lead deposits are Mexico, Russia.
The Lead Concentrate Manufacturing Process is as Below:
Lead Concentrate Raw Materials
Lead is extracted from ores dug from underground mines. More than 60 minerals contain some form of lead, however, only 3 are usually mined for lead production. The most common are called galena. The pure form of galena contains only lead and sulfur, but it’s usually found with traces of other metals in it, including silver, copper, zinc, cadmium, and Sb further as arsenic. Over 95th of all lead mined comes from one of these 3 minerals. However, most deposits of these ores aren’t found alone but mixed with other minerals such as pyrite, marcasite. Therefore, much lead ore is obtained as a byproduct of other metal mining, usually zinc or silver. Only half all lead used yearly is derived from mining, as 0.5 is recovered through recycling, mostly of automobile batteries.
Besides the ore itself, only a few raw materials are necessary for the refining of lead. The ore concentrating process requires pine oil, alum, lime, and Potassium Amyl Xanthate. Limestone or iron ore is added to the lead ore during the roasting process. Coke, a coal distillate, is used to further heat the ore.

Mining the Lead ore
1 the primary step in retrieving lead-bearing ore is to mine it underground. Workers using heavy machinery drill the rock from deep tunnels with heavy machinery, or blast it with dynamite, leaving the ore in pieces. Then they shovel the ore onto loaders and trucks and haul it to a shaft. The shaft of a large mine could also be a mile or more from the drill or blast site. The miners dump the ore down the shaft, and from there it’s hoisted to the surface.
Concentrating the Lead ore
2 after the ore is removed from the mine, it’s treated at a concentrating mill. Concentrating means that to remove the waste rock from the lead. To begin, the ore should be crushed into very small pieces. The ore is ground at the mill, leaving it in particles with diameters of 0.1 millimeters or less. This means the individual granules are finer than table salt. The texture is something like granulated sugar.

Flotation
3 The principal lead ore, galena, is properly known as lead sulfide, and sulfur makes up a substantial portion of the mineral. The flotation process collects the sulfur-bearing portions of the ore, which also contains the valuable metal. First, the finely crushed ore is diluted with water so poured into a tank referred to as a flotation cell. The ground ore and water mixture are called slurry. One percent pine oil or a similar chemical is then added to the slurry in the tank. The tank then agitates, shaking the mixture violently. The pine oil attracts the sulfide particles. Then air is bubbled through the mixture. This causes the sulfide particles to form an oily froth at the highest of the tank. The waste rock, which is called gangue, sinks to the bottom. The flotation process is controlled by means of X-ray analyzers. A flotation monitor in the control room can check the metal content of the slurry using the X-ray analysis. Then, with the aid of a computer, the monitor may adjust the proportion of the chemical additive to optimize recovery of the metal. Other chemicals are added to the flotation cell to help concentrate the minerals. Alum and lime aggregate the metal or make the particles larger. Potassium Amyl Xanthate is also added to the slurry, in order to help the metal particles float to the surface. At the end of the flotation process, the lead has been separated from the rock, and other minerals too, such as metal and copper, have been separated
Filtering
4 after the ore is concentrated in the flotation cells, it flows to a filter, which removes up to 90th of the water. The concentrate at this point contains from 40-80% lead, with large amounts of other impurities, mostly sulfur, and zinc. It’s ready at this stage to be shipped to the smelter. The gangue, or rock that wasn’t mineral-bearing, should be pumped out of the flotation tank. It may be dumped into a pond resembling a natural lake, and when the pond eventually fills, the land can be replanted.
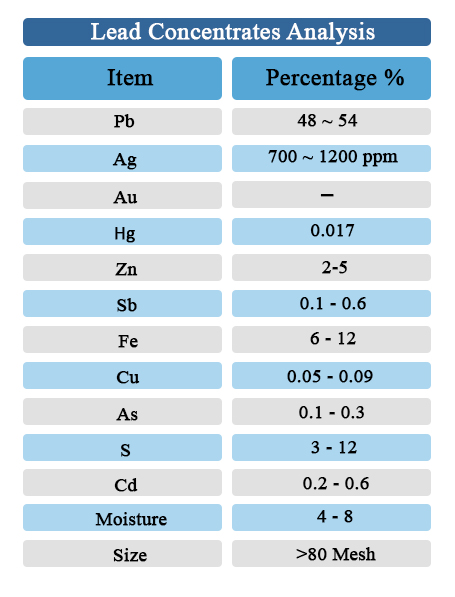
Lead Concentrate Specifications:
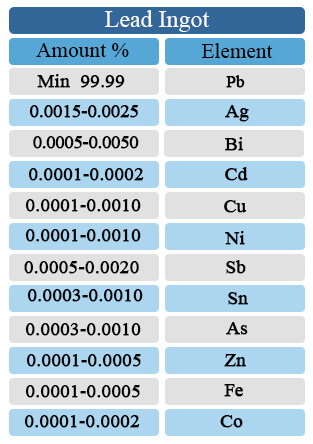
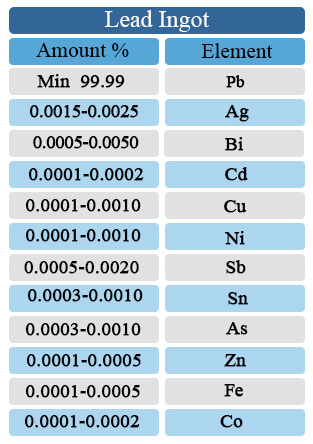
Lead Ingot
The most important industrial use of lead is in the manufacture of lead-acid storage batteries. It’s also used in alloys like melted metals, antifriction metals, solder, and type metal. Shot lead is an alloy of lead, antimony, and arsenic. Lead foil is created with lead alloys. Lead is used for covering cables and as a lining for laboratory sinks, tanks, and so the chambers within the lead-chamber method for the manufacture of oil of vitriol. It’s used extensively in plumbing. as a result of it’s fantastic vibration-dampening characteristics, lead is often used to support heavy machinery. Lead is additionally used as protective shielding against X-rays and radiation from nuclear reactors.
Pig Lead These are uniform 65 lb. to 75 lb. cast bars. Pig lead is usually remelted but can also be used as a ballast product.
Pig is cast with ears to create lifting individual pieces easier. This a simple cast product, however, has a variety of applications.
Easy to handle and simple to stack Pure leads pig is used as ballast or trim weight for jobs requiring concentrated weights, such As marine applications barge ballast, ship ballast and more.
Pure Lead stocks 99.99% and 99.97% pig lead for immediate shipment.
Ingot Lead is otherwise known as Caulking Lead. These are 25 lb. bars with 5 5 lb. round lead sections.
This product is mostly employed by plumbers to caulk pipe joints.
Other uses are for ballast and other weight/counterweight applications.
Lead Ingots are simple to melt. They’re very clean and nice for casting fishing weights, casting bullets or mistreatment anyplace else 99.9% lead is required.
Pure Lead products use the finest quality clean lead. Call us today for a quote.
Our pig lead Ingots and Caulking Lead are always in stock and can ship same day if needed.
Lead Ingot anlysis:

The Lead Ingot manufacturing process:
- Mining the ore
- Concentrating the ore
- Flotation
- Filtering
- Roasting the ore
- Blasting
- Refining
Roasting the ore
- The lead concentrate fresh from the filter needs to be further refined to remove the sulfur. After the concentrate is unloaded at what’s called the mold plant, it’s mixed with other lead-bearing materials and with sand and limestone. Then the mixture is spread on a moving grate. Air which has been heated to two, 550F (1,400C) blows through the grate. Coke is added as fuel, and also the sulfur in the ore concentrates composts to sulfur dioxide gas. This sulfur dioxide is an important byproduct of the lead refining process. It’s captured at a separate acid plant and converted to sulfuric acid, which has several uses. After the ore has been roasted in this way, it fuses into a brittle material called sinter. The sinter is mostly lead oxide, but it can also contain oxides of zinc, iron, and silicon, some lime, and sulfur. As the sinter passes off the moving grate, it’s broken into lumps. The lumps are then loaded into the furnace.
Lead Blasting
- The inter falls into the top of the blast furnace, along with coke fuel. A blast of air come through the lower part of the chamber, combusting the coke. The burning coke generates a temperature of about 2,200F (1,200C) and produces carbon monoxide. The carbon monoxide reacts with the lead and other metal oxides, producing molten lead, nonmetallic waste slag, and carbon dioxide. Then the molten metal is drawn off into dressing kettles or molds.
Lead Refining
- The molten lead as it comes from the blast furnace is from 95-99% pure. It’s called at now base bullion. It should be further refined to get rid of impurities, because commercial lead should be from 99-99.999% pure. To refine the bullion, it is kept in the dressing kettle at a temperature just above its melting point, about 626F (330C). At this temperature, any copper left in the balloon rises to the top of the kettle and forms a scum or dross which can be faithless off. Gold and silver can be removed from the bullion by adding to that a small quantity of zinc. The gold and silver dissolve additional easily in zinc than in the lead, and once the balloon is cooled slightly, a zinc dross rises to the top, bringing the other metals with it.
Lead Costing
- when the lead has been sufficiently refined, it’s cool and cast into blocks which might weigh as much as a ton. This is the finished product. Lead alloys may also be produced at the smelter plant. During this case, metals add to the molten lead in precise proportions to produce a lead material for specific industrial uses. For example, the lead commonly used in car batteries, and also for pipe, sheet, cable sheathing, and ammunition, alloys with antimony because this increases the metal’s strength.

Lead Byproducts/Waste
Lead refining produces several byproducts. The gangue, or waste rock, accumulates as the ore is concentrated. Most of the minerals have been removed from the rock, so this waste isn’t considered by the industry to be an environmental hazard. It can be pumped into a disposal pond, which resembles a natural lake. Sulfuric acid is the major byproduct of the smelting process. Sulfur dioxide gas is released once the ore is roasted at the sinter plant. To shield the atmosphere, fumes and smoke area unit captured, and the air released by the plant is first cleaned. The sulfur dioxide is collected at a separate acid plant and converted to sulfuric acid. The refinery can sell this acid as well as its primary product, the lead itself.
Air pollution can result from lead processing as well. The smelter requires a bag house, that is, a separate facility to filter and vacuum the fumes so lead isn’t released into the atmosphere. Nevertheless, lead particles do reach the atmosphere, and in the U.S., federal rules attempt to control how much is allowable. Most of the solid stuff produced by the smelting process is a dense, glassy substance called slag. This contains traces of lead as well as zinc and copper. The slag is additional toxic than the gangue, and it should be stored securely and monitored so that it doesn’t escape into the environment or come in contact with populations.
The Future of Lead Ingots
New developments in the lead industry seem aimed less at improvements in the manufacturing process than towards finding new uses for the lead itself. Since a large proportion of the lead mined and recycled is sold to the automotive industry for batteries, lead producers are quite dependent on the health of the car industry. But lead producers are interested in finding new applications for the lead to give them more market stability.
One recent new application for lead is a lead-fiberglass laminate. Lead sheeting can be laminated between gypsum and fiberglass, forming a superior duct material that helps isolate noise. If this is used in an air conditioning unit, for example, it effectively dampens the din of the machine. Another prospective market for lead is in nuclear waste containment. Safely storing radioactive material is a growing concern around the world. The lead industry is researching canisters made of titanium with an inner layer of lead or lead and plastic, contending that a one-inch layer of lead could add 880 years to the life of a properly buried container. And looking for the cars of the future, researchers in the several countries have been studying ways of improving lead-acid battery technology in order to power electric cars.
Comments Off on Lead with 631 visit